7 Which of the Following Accurately Describes the Ph Scale
The pH scale runs from 0 most acidic to 14 most basic with 7 as a neutral. The lower the concentration of hydrogen ions the higher the acidity is.

The Four Things You Need To Know About Soil Ph Finegardening Soil Ph Fine Gardening Soil
Solutions with a pH of 7 are neutral.
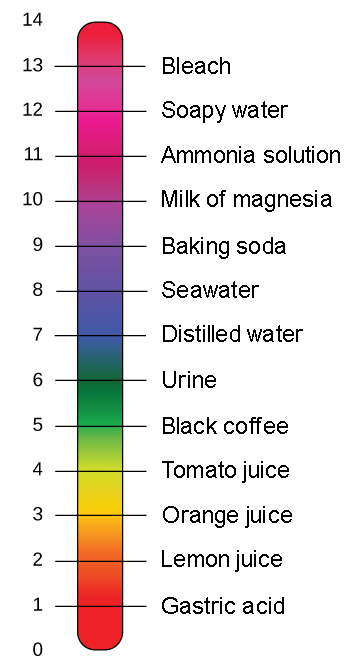
. A pH of 7 is neutral. A pH value is used to describe a water-based solution. A scale used to define the levels of hydrogen H or hydroxide OH ions in a solution.
Bases have pH values higher than 7. The pH scale is logarithmic and as a result each whole pH value below 7 is ten times more acidic than the next higher value. Correct answer to the question Which of the following accurately best describes the ph scale.
Explore thepH ScaleSelect the Macro tab. A Drag the pH sensor into the solution to see the pH reading. If pH 7 then the solution is acidic.
Which of the following accurately best describes the ph scale. Which of the following accurately describes the pH scale. PHs of less than 7 indicate acidity whereas a pH of greater than 7 indicates a base.
17 Which of the following accurately describes the pH scale. There are more H than OH-in an acidic solution. Solutions having a value of pH ranging 0 to 7 on pH scale are termed as acidic and for the value of pH ranging 7 to 14 on pH scale are known as basic solutions.
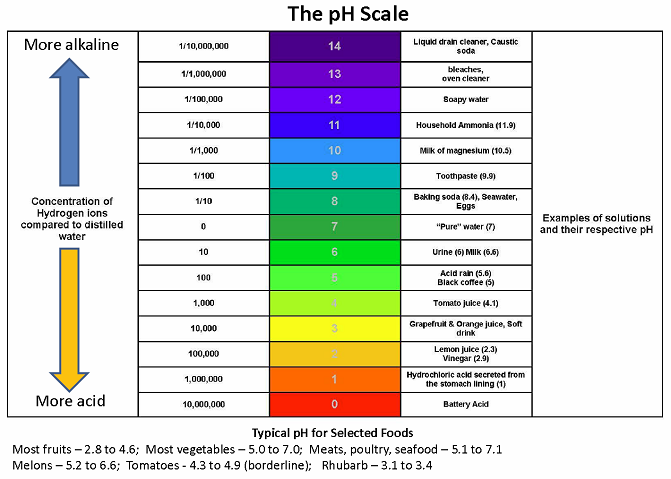
The pH scale ranges from 0 to 14. The following accurately describes the pH scale. Solutions with a pH greater than 7 are alkaline.
Investigate the pH of each of the substances found under the drop-down menu. The lower the pH is the more basic the solution is. Abbreviation meaning potential for hydrogen pH Scale.
For example pH 4 is ten times more acidic than pH 5 and 100 times 10 times 10 more acidic than pH 6. A solution that is between 0-6 on the pH Scale. 1 The pH scale.
Who are the experts. Compare the pH scale on the left-hand side of the screen to the pH scale below. Week 7 Laboratory PhET Assignment.
The range goes from 0 - 14 with 7 being neutral. We review their content and use your feedback to keep the. At 100C a pH value of 614 is the new neutral point on the pH scale at this higher temperature.
Experts are tested by Chegg as specialists in their subject area. The pH of a solution varies from 0 to 14. The pH scale is centered on 7 - meaning that a solution with a pH of 7 is perfectly neutral neither acidic nor basic.
PH of Acids and Bases. If universal indicator is added to a solution it changes to a. If pH 7 then the solution is basic.
This problem has been solved. Knowing the dependence of pH on H we can summarize as follows. A pH less than 7 is acidic.
Solutions with a pH less than 7 are acidic. PH values have a negative logarithmic relationship to the H ion concentration in the solution. Amphoteric ampiprotic substances have very high pH values.
At pH 7 the substance or solution is at neutral and means that the concentration of H and OH - ion is the same. PH is usually but not always between 0 and 14. The blood in your veins is slightly alkaline pH 74.
View more similar questions or. Define pH and describe the pH scale and what the pH scale measures. Other questions on the subject.
A The pH scale runs from 0 most basic to 14 most acidic with 7 as a neutral. Which of the following accurately best describes the ph scale. Something with a pH of 5 would be ________.
B The pH scale runs from 0 most acidic to 14 most basic with 7 as a neutral. Neutral water has a pH of 7. Micro range pH test paper.
Water that has more free hydrogen ions is acidic whereas water that has. Orange juice is mildly acidic pH approximately 35 whereas baking soda is basic pH 90. If pH 7 then the solution is neutral.
High quality pH meters can be expensive. 01 or less depending on the meter. Ranges from 0 acidic to 14 basicalkaline with 7 being neutral.
Anything below 70 ranging from 00 to 69 is acidic and anything above 70 from 71 to 140 is alkaline. Acids have pH values below 7. A pH greater than 7 is basic.
We recommend checking if there is one available at your local high school chemistry laboratory before purchasing. The environment in your stomach is highly acidic pH 1 to 2. PH is a measure of how acidicbasic water is.
Label the one below as acidic and basic. In general a small pH value describes a solution that is acidic and a larger pH value describing solutions that are less acidic more basic. If pH 7 the solution is acidic.
The pH scale does not have an upper nor lower bound. PH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water.

Alkaline Acidic Foods Chart The Ph Spectrum Acidic Food Chart Acidic And Alkaline Foods Health And Nutrition

The Ph Scale Biology For Non Majors I
What Is Ph College Of Agriculture Forestry And Life Sciences Clemson University South Carolina

The Ph Levels Of The Beans Remove Toxins Healthy Water Drinks Banner Printing
Belum ada Komentar untuk "7 Which of the Following Accurately Describes the Ph Scale"
Posting Komentar